How do hormonal changes that happen during and after pregnancy change the brain? Are these changes associated with changes in behavior?
The peripartum period (pregnancy and postpartum) is a time of profound changes in both hormones and behavior. While some changes in behavior are adaptive (e.g., bonding, displaying parental behaviors), other behavioral changes reflect low mood and increased anxiety. This is significant, as an estimated 15-20% of people experience peripartum psychological complications, including depression and/or anxiety. Although it is assumed that hormonal changes associated with pregnancy and birth are related to the symptoms of peripartum depression and anxiety, how these hormonal changes impact the brain is poorly understood. Using animal models, we can isolate and manipulate these hormonal changes to ask whether they influence changes in the brain and in behavior. Ongoing experiments in my lab are looking at peripartum plasticity in oxytocin, dopamine, and serotonin signaling and the resulting impact on behavioral indices of anxiety, depression, motivation, and sleep. A better fundamental understanding of these processes is vital to understanding the neurobiological components of peripartum mood, anxiety, and sleep disorders, and may point to new therapeutic targets in people.
How does sex experience change the brain? Are these changes the same in males and females?

One of our overarching research interests is understanding the neurobiology of naturally-motivated behaviors. Specifically, we are interested in the effects of rewarding experiences on future behavior, focusing on how rewarding experiences that occur as part of an individual’s everyday life can lead to long-lasting changes in the brain. To that end, we use sex behavior in Syrian hamsters as a model to investigate the neurobiological mechanisms of natural reward and brain plasticity following rewarding experiences.
Previous research has demonstrated that sex experience increases the density of dendritic spines on neurons of the nucleus accumbens, a brain region critical for reward and motivation. Put another way, sex experience changes the physical structure of neurons in the nucleus accumbens, making it possible for more synapses form. Although this has been shown to occur in both males and females of several species, and has important implications for the response to future rewarding experience (either natural or synthetic), the molecular events that lead to this structural plasticity are not known. Furthermore, whether the same signaling mechanisms mediate this change in both sexes are not known. In my lab, we are using molecular biology and behavioral pharmacology techniques to try to dissect the neural mechanisms underlying this neuroplasticity.
What are the neural mechanisms of aggression in females?
Although many people think of aggression as a negative or undesirable emotion, it is a typical part of many species’ repertoire of social behaviors and can have rewarding consequences. In fact, aggressive behaviors typically serve an adaptive function– these purposeful and controlled behaviors serve to warn other individuals of perceived breaches in social contracts, typically with the goal of dispersing conflict before it escalates into physical violence. Aggression becomes maladaptive when it escalates inappropriately or impulsively into violence. This type of aggression, characterized by a loss of behavioral control, presents as a symptom of numerous psychiatric disorders. Despite this, the neurobiology of aggression is poorly understood.
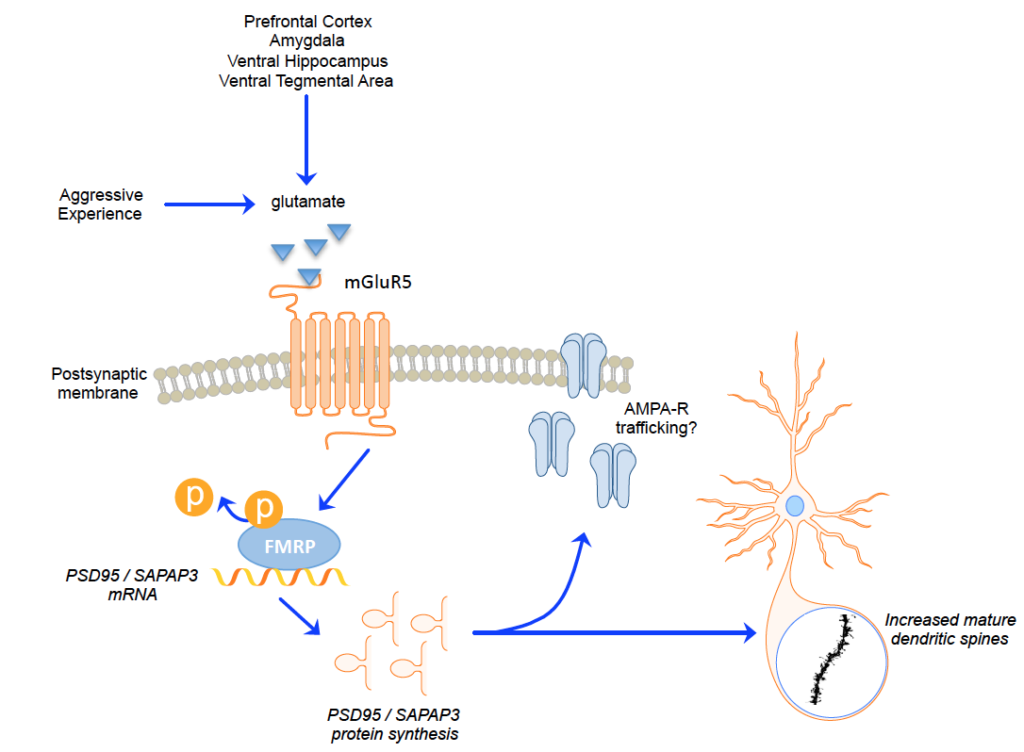
Previous research in my postdoctoral laboratory demonstrated that when female hamsters repeatedly experience brief aggressive encounters, the amount of time it takes them to attack a non-aggressive intruder in their cage significantly decreases across time. Put another way, their aggressive behavior escalates into a maladaptive response, modeling the “short fuse” seen in pathological, impulsive aggression. As with repeated sex experience, repeated aggressive encounters are associated structural changes in the nucleus accumbens. However, the intracellular signaling events leading to these neural changes were unknown. We hypothesized that a particular molecular signaling pathway, the Fragile X Mental Retardation Protein (FMRP) pathway, mediated the neural and behavioral changes we saw in female hamsters following repeated aggressive encounters. Using a mixed-methods approach (behavior analysis, molecular biology, and pharmacology), we built evidence for a model in which aggressive experience activates mGluR5 receptors on neurons in the nucleus accumbens, leading to a rapid decrease in the phosphorylation of FMRP and long-lasting increases in the transcription and translation of post-synaptic scaffolding proteins. With repeated experiences, these changes may result in a change in neural signaling that mediate the escalated aggressive behavior seen in future encounters.
What are the neural mechanisms of sexual attraction/copulation? Are these circuits susceptible to plasticity?
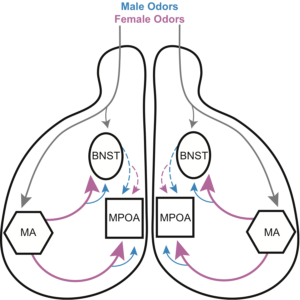
My doctoral research used excitotoxic micro-lesions and neuroanatomical tract tracing to investigate the neural circuitry underlying appetitive and consummatory aspects of reproductive behavior in male hamsters. Specifically, I demonstrated that the connections between the medial amygdala and medial preoptic area (MPOA) are necessary for copulation, but not attraction; in contrast, the connections between the medial amygdala and bed nucleus of the stria terminalis (BNST) are necessary for attraction, but not copulation. Interestingly, giving animals sexual experience prior to lesioning the BNST or MPOA ameliorated the behavioral effects of the lesions, suggesting that sexual experience can mitigate the necessity of these brain areas for producing reproductive behaviors. The mechanism of this plasticity is unknown. Ongoing experiments in my lab are testing the hypothesis that plasticity following sex experience is mediated by increased connectivity between the BNST/MPOA and the nucleus accumbens.